An interdisciplinary team from RWTH Aachen University Hospital in Germany has developed a new method to image, diagnose, and stage kidney fibrosis. The increased production of the protein elastin in diseased kidneys proved key to this finding. By using an MRI contrast agent specific to elastin, the investigators demonstrated the use of their technique for longitudinal diagnosis and monitoring of kidney fibrosis, which is currently not possible in a noninvasive manner.
Chronic kidney disease (CKD) is a growing healthcare burden worldwide. The best current predictor of the progression of CKD is fibrosis -- the uncontrolled deposition of extracellular matrix protein in response to injury, which in itself can hamper tissue function. In CKD, kidney function is often assessed using the glomerular filtration rate (GFR), but this often does not accurately reflect the extent of fibrosis. Currently, the only method to assess fibrosis is via invasive tissue biopsies. This places a severe limitation on CKD diagnosis and staging, as fibrosis progression cannot be easily monitored over time. Consequently, development of antifibrotic drugs is also limited.
Alternatives to biopsies have already been sought, including serum and urinary biomarkers of kidney fibrosis. However, these are often not organ-specific, and they are more indicative of organ function, rather than fibrosis progression. To date, there is no tool for longitudinal diagnosis of kidney fibrosis.
Going beyond biopsy
Researchers led by Dr. Peter Boor, PhD, and Twan Lammers, PhD, have now identified that the protein elastin is significantly overproduced in fibrotic, diseased kidneys and can serve as a useful biomarker of fibrosis. Using a comprehensive list of 10 different mouse and rat models of renal fibrosis, they showed that elastin was significantly overexpressed in the medulla and the cortical interstitium of the kidney, the latter being particularly suitable for molecular imaging (Science Translational Medicine, 3 April 2019, Vol. 11:486, eaat4865).
Likewise, the researchers identified significant elastin overexpression in 146 samples from human patients, which encompassed all major renal diseases. They used this increase in elastin production in diseased and fibrotic kidneys as a basis to develop a novel imaging method to diagnose kidney fibrosis.
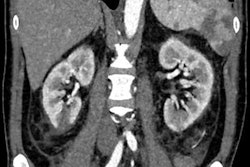
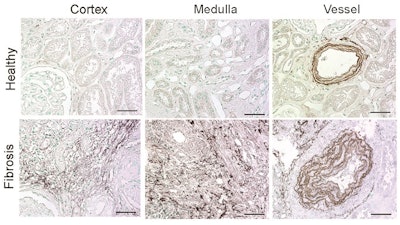 Using the ESMA MRI technique, the researchers identified that elastin is overproduced in diseased kidneys (bottom row) compared with healthy kidneys (top row). Image courtesy of Sun et al, Science Translational Medicine.
Using the ESMA MRI technique, the researchers identified that elastin is overproduced in diseased kidneys (bottom row) compared with healthy kidneys (top row). Image courtesy of Sun et al, Science Translational Medicine.The group utilized the imaging agent ESMA, an elastin-specific MRI contrast agent that has already been demonstrated to target elastin when imaging atherosclerotic plaques or liver fibrosis. MR imaging with ESMA complemented the team's previous findings and showed that animal and human kidneys with fibrosis and upregulated elastin expression can be noninvasively monitored by this new technique.
The researchers also analyzed whether their method could be used to monitor intervention with antifibrotic drugs. Using two different treatment approaches and different animal models, they showed that antifibrotic agents reduced elastin expression and that this was closely reflected by ESMA MRI. The authors also showed that 95% of the imaging agent was eliminated 72 hours after administration, allowing longitudinal monitoring of fibrosis progression, as shown in a mouse model of fibrosis over a period of 21 days.
Importantly, the authors also compared their approach to the diagnostic tool most commonly used to assess kidney function, the GFR. In a model of reversible kidney disease, after an initial disease period, mice appeared to recover over 14 days, as indicated by normalization of their GFR. However, the researchers' imaging method showed that fibrosis remained, as elastin remained elevated. This demonstrated that functional recovery differs greatly from the overall structural damage in kidney disease.
Taking ESMA to trial
The technique provides a potential tool for longitudinal diagnosis and staging of kidney disease, but the authors noted two considerations for its translation. First, a clinical trial should be designed to ascertain the sensitivity and specificity of ESMA MRI in many patients and compare it with the gold standard, biopsies. The extensive animal model and patient samples that the authors used for validation of their technique are an excellent basis for this trial.
Second, it must be confirmed that the contrast agent (which contains gadolinium) does not lead to side effects, in particular gadolinium-associated nephrogenic systemic fibrosis. To combat this, it could be possible to produce ESMA with other, safer contrast agents, such as gallium or indium.
The elastin MRI diagnostic tool for kidney disease has many benefits over current methods, including specificity for fibrosis and the organ of interest, noninvasiveness, the ability to perform longitudinal monitoring over time, its quantitative nature, and its high sensitivity. The team also concluded that a specific group of cells, PDGFR-β+ myofibroblasts, are the main culprit for elastin deposition, potentially opening an avenue for future therapy development.
Ultimately, this imaging breakthrough may lead to an improvement in kidney disease diagnosis and monitoring, which in turn could improve the development and translation of novel, specific, and effective treatments.
Gregor Skeldon is a doctoral student contributor to Physics World. He is studying bioengineering at Heriot-Watt University in Edinburgh, U.K.
© IOP Publishing Limited. Republished with permission from Physics World, a website that helps scientists working in academic and industrial research stay up to date with the latest breakthroughs in physics and interdisciplinary science.