The sensitivity and specificity of artificial intelligence (AI) algorithms must be compared with that of experienced readers who follow strict mammography viewing protocols, according to breast imaging pioneer Dr. László Tabár, who urges caution when comparing the performance of AI with that of radiologists.
AI providers should specify which cancer subtypes are being detected or missed by their programs, emphasized Tabár, professor emeritus of Uppsala University in Sweden.
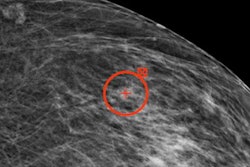
 Dr. László Tabár.
Dr. László Tabár."I am truly enthused by the potential benefit of AI, since it may bring breast cancer detection at mammography screening to the same high level everywhere," he told AuntMinnieEurope.com.
"I encourage the AI innovators to perform precision medicine-type comparisons, i.e., assess the performance of AI against skilled radiologists who enlarge each image when viewing mammograms, and also disclose the strengths and weaknesses of their algorithms for each breast cancer subtype classified according to its site of origin," he added.
Reducing breast cancer death hinges on strict reading protocols and finding the cancers that are potentially harmful, with the long-term outcome of the breast cancer patient depending largely on the site of origin of the disease. But there is still a wide variation in how radiologists view mammograms and whether the breast cancers are classified according to their site of origin, Tabár explained.
Detecting killer cancers
Since 1986, Tabár has helped train around 25,000 radiologists, and yet he notes that considerable discrepancies in reading skills remain. He isn't surprised that AI can outperform the inexperienced reader, but would it outperform the educated one?
"AI has the potential to help minimize the current variability among readers," he said. "I would like to warn about the risks of inappropriate AI evaluation and help ensure that AI finds the cancers that have imminent harmful potential."
To decrease mortality, screening has to decrease the advanced cancer rate. The site of origin of breast cancer, reflected in the tumor's mammographic appearance (or imaging biomarkers) is directly related to the long-term patient outcome, according to Tabár.
Around 75% of cancers originate from the terminal ductal lobular units (TDLUs) and should be termed acinar adenocarcinoma of the breast (AAB). These are stellate or spherical/oval-shaped on mammograms. Both the radiologists and AI need to find these cancers when they are smaller than 15 mm because the 26-year survival of these cancers is 92%-95% -- a drastic improvement from the 1970s. But accomplishing this goal often depends on whether the breast structure is adipose or dense fibroglandular tissue, he continued.
Approximately 20% of cancers develop from the major milk ducts and should be termed ductal adenocarcinoma of the breast, and these are probably of stem cell origin. While the majority present on the mammogram as microcalcifications that are easy to detect, a large number do not calcify until they are extensive.
"Such factors explain the dismal 70% survival, which hasn't improved in recent decades despite screening and use of modern therapeutic regimens. Finding these cancers earlier is an important challenge for AI," Tabár said.
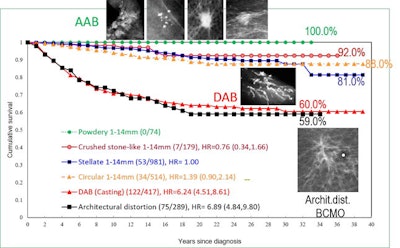 Cumulative 39-year survival of breast cancer cases by imaging biomarkers: 1-14 mm acinar adenocarcinoma of the breast (AAB), all cases of ductal adenocarcinoma of the breast (DAB) and breast cancers of mesenchymal origin (BCMO), diagnosed in Dalarna County, Sweden, 1977-2014. The goal is to move the DAB and BCMO curves upward to save more lives. Graph courtesy of Drs. László Tabár, Amy Ming-Fang Yen, and Tony HH Chen.
Cumulative 39-year survival of breast cancer cases by imaging biomarkers: 1-14 mm acinar adenocarcinoma of the breast (AAB), all cases of ductal adenocarcinoma of the breast (DAB) and breast cancers of mesenchymal origin (BCMO), diagnosed in Dalarna County, Sweden, 1977-2014. The goal is to move the DAB and BCMO curves upward to save more lives. Graph courtesy of Drs. László Tabár, Amy Ming-Fang Yen, and Tony HH Chen.The remaining 5% of breast cancer cases are of stem cell origin too, arising from the mesenchymal stem cells through the complex process of mesenchymal-epithelial transition (MET). This subtype of breast cancer of mesenchymal origin (BCMO) is currently termed diffusely infiltrating lobular carcinoma. These malignancies are usually between 5 cm and 10 cm in size at the time of detection, and they often have a spiderweb-like appearance on the mammogram (architectural distortion) that may be very difficult to detect, he said. The long-term survival of this breast cancer subtype is 60%, a figure that hasn't improved over the last 40 years.
"We believe there is no mammographically detectable early phase in this subtype, since most of the fibrous connective tissue of the organ is involved in the malignant process, which, in most cases, appears to evolve over a short period of time. Therefore, both the DAB and BCMO present with a massive burden of malignant tissue," Tabár noted.
He believes that for true precision medicine, physicians and AI developers alike shouldn't use general terms such as "breast cancer" when reporting their results. Instead, disclosure should include the percentage of AAB (stellate and circular) tumor masses found in the size range of 1-14 mm and whether DAB and BCMO can be found earlier, when they are still confined to the breast.
Advice to radiologists
To radiologists, Tabár recommends the following: When viewing mammograms, make every image larger and darker.
Mammography reading carries great responsibility, and if radiologists don't enlarge and darken images, they will miss more cancers than those who do. Consequently, if AI is compared with readers who don't follow this protocol, then evaluators are going to believe AI is better, he added.
"I'm not interested in reading about evaluating AI against radiologists who don't enlarge the picture. AI has to beat me and my students who view mammograms in the way they should be viewed," Tabár noted.
"We must bring every reader to the same high level of sensitivity and specificity, and the development of reliable AI programs can help us in this effort. It would be desirable if women could go anywhere for screening and benefit from the same high, uniform quality of mammography screening."
To read more on this topic, you can download two presentations by Tabár about mammography reading protocols and cancer subtypes at the following links: Dropbox lecture 1, and Dropbox lecture 2.