Deep-learning-enhanced image reconstruction algorithms quantify tissue on abdominal CT more reliably and thus improve image quality, according to a German study published on 16 February in European Radiology.
In fact, using deep learning-based reconstruction extracts more radiomic features from CT images without an ensuing uptick in radiation dose, wrote a team led by Drs. Florian Michallek and Paul Jahnke of the Charité - Universitatsmedizin Berlin.
"Deep-learning reconstruction [DLR] enhances image quality for radiomics and more than doubles the feature yield at doses that are typically used in clinical CT imaging," Michallek's group reported.
Inconsistent tissue characterization within CT images "contributes significantly to the poor stability of radiomics features," the authors noted. So Michallek and colleagues compared the performance of a deep-learning reconstruction algorithm (Canon Medical Systems, Advanced intelligent Clear-IQ Engine, or AiCE) for extracting radiomics features from CT exams to the following other techniques:
- Filtered back projection (FBP)
- Hybrid iterative reconstruction (AIDR 3D)
- Model-based iterative reconstruction (FIRST)
"Deep-learning reconstruction has been reported to be effective in reducing noise and preserving noise texture, in contrast to previous FBP and iterative reconstruction algorithms," Michallek told AuntMinnieEurope.com. "We therefore expected that DLR might have significant potential to improve radiomics feature extraction from CT images."
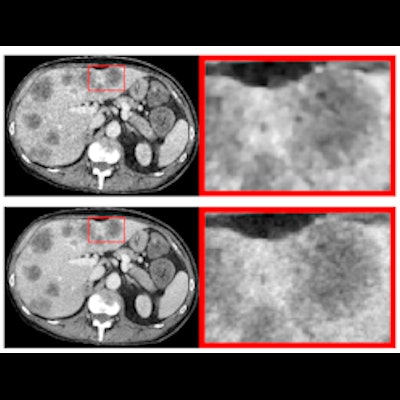
The team used a phantom that simulated a 65-year-old patient with liver metastases, scanning it at 18 different doses (range, 0.2 mGy to 4 mGy) with 20 scans per dose. The group reconstructed the images with each of the four techniques, extracting 93 radiomic features from 24 regions of interest across three simulated tissue categories: normal liver, metastatic core, and metastatic rim. The AiCE technique performed best.
%{[ data-embed-type="image" data-embed-id="6555227764e6b8cb92446be6" data-embed-element="span" data-embed-alt="CT images acquired with 0.2 and 4 mGy and reconstructed with four different reconstruction algorithms. FBP is filtered back projection. AIDR 3D (Adaptive Iterative Dose Reduction 3D), FIRST (Forward projected model-based Iterative Reconstruction SoluTion), and AiCE (Advanced intelligent Clear-IQ Engine) are the manufacturer’s implementation of hybrid iterative reconstruction, model-based iterative recon- struction, and deep learning reconstruction, respectively. Figure courtesy of European Radiology and Dr. Florian Michallek and Dr. Paul Jahnke." data-embed-src="https://img.auntminnieeurope.com/files/base/smg/all/image/2022/02/ame.2022_02_22_16_39_8269_2022_02_23_Michallek_2.png?auto=format%2Ccompress&fit=max&w=1280&q=70" data-embed-caption="CT images acquired with 0.2 and 4 mGy and reconstructed with four different reconstruction algorithms. FBP is filtered back projection.<br>AIDR 3D (Adaptive Iterative Dose Reduction 3D), FIRST (Forward projected model-based Iterative Reconstruction SoluTion), and AiCE (Advanced intelligent Clear-IQ Engine) are the manufacturer’s implementation of hybrid iterative reconstruction, model-based iterative recon- struction, and deep learning reconstruction, respectively. Figure courtesy of European Radiology and Dr. Florian Michallek and Dr. Paul Jahnke." data-embed-width="616" data-embed-height="400" ]}%| Performance of four different CT image reconstruction techniques | ||||
| Measure | FBP | AIDR 3D | FIRST | AiCE |
| Median fraction of consistent features across all doses | 6% | 8% | 6% | 22% |
| Adequate discrimination power of key features | 48% | 82% | 84% | 92% |
| Feature repeatability | 52% | 20% | 17% | 39% |
The group also found that FBP, AIDR 3D, and FIRST combined consistency, discriminative power, and repeatability for only 5% of key features, while AiCE did this for 13% of features at doses above 1 mGy and for 17% of features at doses at or greater than 3 mGy. AiCE was also the only technique that extracted higher-order features, the team reported.
Are there barriers to implementing this type of deep-learning reconstruction into clinical practice? Perhaps, but it's likely just a matter of time before this technique is incorporated into the radiology department, Michallek said.
"The DLR algorithm we used is commercially available and thus accessible to anyone with comparable scanner technologies," he noted. "However, many clinical scanners are not yet equipped with such technologies, which in turn can limit accessibility."
In any case, the research could support retrospective data collection and prospective implementation of CT protocols for radiomics, according to Michallek.
"Our findings have three main implications," he told AuntMinnieEurope.com. "FBP and IR yield inadequate image quality for the majority of radiomics features due to inconsistent tissue characterization, low discriminative power, or low repeatability; deep learning techniques can improve image quality for radiomics, and DLR should preferably be used when available; and despite superiority of DLR in our study, we see significant potential to further increase the yield of reliable features, possibly by employing dedicated deep-learning image processing techniques for radiomics."
The study findings could also improve patient care, he noted.
"Deep-learning reconstruction has the perspective of enabling reduced dose imaging and improved application of radiomics," Michallek said. "These advances may lead to reduced patient risk from radiation exposure and potentially improved diagnostic precision through the use of radiomics models."