Evidence about the use of MRI in patients with a heart-valve prosthesis or an annuloplasty ring remains inconclusive, but the latest data support the hypothesis of a low or negligible interaction with MRI, according to new research presented at the recent European Society of Cardiovascular Radiology (ESCR) congress in Milan.
"If confirmed by other larger studies, patients carrying a metallic heart prosthesis may safely undergo MRI under controlled conditions," noted Dr. Giulia Lastella, from the postgraduate school in the Radiodiagnostic Università degli Studi di Milano, Policlinico San Donato. "Metallic heart prostheses such as a heart-valve prosthesis and annuloplasty rings in the patient's body are still a contraindication to MRI."
In March 2017, Lastella and colleagues conducted a systematic review of the literature using Medline and Embase for original articles published in English on the safety of MRI in patients with a heart-valve prosthesis or annuloplasty ring. They also included articles evaluating the effect of MRI on such prostheses in vitro. The keywords were MRI, heart prosthesis, valve, safety, in vitro, and adverse events.
Two independent readers reviewed all abstracts for eligibility, Lastella explained. The full text of papers deemed eligible was retrieved, and the references of the retrieved papers were searched by hand for additional studies. The number and age of patients, type and size of the device, the magnetic field strength, and major clinical adverse events were extracted.
Key findings
The search retrieved 631 studies, 13 being analyzed for a total of 226 prostheses (128 in vivo and 98 in vitro). All studies had a prospective design: Six articles evaluated only a prosthesis in vivo (122 prostheses), six articles evaluated only a prosthesis in vitro (93 prostheses), and one was mixed (six prostheses in vivo and five in vitro). In vivo, 16 valves were tested at 0.2 tesla, nine at 0.35 tesla, 120 at 1.5 tesla, and 24 at 2.35 tesla, making a total of 169 MRI evaluations. In vitro, 98 valves were tested at 1.5 tesla, nine at 2.35 tesla, and 23 were tested at 4.7 tesla, making a total of 130 MRI evaluations.
"Theoretically, there is a potentially dangerous interaction between metallic parts of heart-valve prosthesis and the static magnetic field (Lenz effect)," Lastella noted. "However, for magnetic fields up to 3 tesla, deflection forces were mathematically considered to be less than the force exerted on the valves by the heart, making the Lenz effect negligible."
Evaluating the magnetic deflection related to exposure to a 1.5- or a 2.35-tesla static magnetic field, no side effects were registered. Ex vivo studies on prosthetic valves and annuloplasty rings in MRI up to 1.5 tesla demonstrated there is no hazard of movement or dislodgment of the implant, with minimal temperature change from 0.5° C to 0.8° C and deviations less than 2 torque, confirming the safety of MRI, the authors stated.
Just one valve -- the Starr-Edwards Pre 6000 (American Edwards) heart valve -- tested up to 1.5 tesla was considered unsafe because of the possibility of movement or dislodgment during the procedure with field strengths greater than 0.35 tesla, but actually it is considered safe up to 1.5 tesla, they pointed out.
"Some of these valves were studied ex vivo at field strengths of 0.35 and 1.5 tesla with no particular alterations of deflection and distortion, except for the Starr-Edwards Pre 6000 and the Starr-Edwards Aortic 2320 valves, which have been tested some years later, demonstrating that MRI is safe," they concluded. "Some prosthetic heart valves and annuloplasty rings were tested too: Just one implant, Carpentier-Edwards Physio Annuloplasty Ring (mitral model 4450), showed a very low deflection angle and torque, but it is considered safe up to 3 tesla."
Safety of other implants
In another Italian presentation at ESCR 2017, a group headed by Moreno Zanardo, a doctoral candidate in integrated biomedical research at the University of Milan, reported the findings of a safety review of patients with pacemakers or implantable cardioverter defibrillators (ICDs).
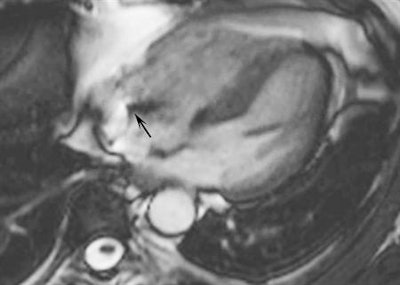 Cardiac MR of a 57-year-old man with a pacemaker. The oblique four-chamber cine steady state with free precession image is not hampered by artifacts from the impulse generator of the pacemaker. Only a small artifact generated by the electro-catheter is visible in the right atrium (black arrow). Image courtesy of Moreno Zanardo.
Cardiac MR of a 57-year-old man with a pacemaker. The oblique four-chamber cine steady state with free precession image is not hampered by artifacts from the impulse generator of the pacemaker. Only a small artifact generated by the electro-catheter is visible in the right atrium (black arrow). Image courtesy of Moreno Zanardo.Of the 493 articles, 53 were analyzed (four randomized, 6,237 exams in 5,394 patients). The devices were MRI nonconditional in 2,889 patients, conditional in 1,708 patients, and mixed in 797 patients. The field strength was 0.2 tesla in one study, 0.5 tesla in three, 1.5 tesla in 45, 2 tesla in one, 3 tesla in two, and mixed in one. The examination was thoracic in 27 studies, nonthoracic in 16, and mixed in 10. No fatal events occurred.
Five studies reported clinically relevant adverse events: atrial arrhythmias (six patients), intolerable heating (five patients), perforation (four patients), lead dislodgements (three patients), and generator failure with immediate replacement (one patient), making a total of 19 adverse events, seven of which involved conditional ICDs, nine nonconditional, and three undefined ICDs. Technical adverse events were mainly power-on reset and battery voltage reduction. Two studies showed significant changes of atrial sensing, four of ventricular sensing, three of atrial pacing capture threshold (PCT), four of ventricular PCT, six of atrial lead impedance, seven of ventricular lead impedance, and nine of battery voltage.
![Cardiac MR of a 59-year old patient carrying an implantable cardioverter defibrillator (ICD). The cine steady state with free precession sequence (time of repetition [TR] 50 ms, time to echo [TE] 3.3 ms, flip angle 80°) acquired along the four-chamber view (a) was largely impaired by a large concentric artifact (asterisk in a) not allowing for segmenting both ventricles. The corresponding cine gradient echo fast low-angle shot sequence (b) (TR 49 ms, TE 1.5 ms, flip angle 15°) shows a strongly reduced artifact generated by the ICD not impairing the evaluation of both ventricles. The artifact generated by the electro-catheter is visible in the right atrium in both images (white arrows in a and b). Image courtesy of Moreno Zanardo.](https://img.auntminnieeurope.com/files/base/smg/all/image/2017/11/ame.2017_11_15_16_20_6001_2017_11_13_MRI_insider_pic11_20171115163055.png?auto=format%2Ccompress&fit=max&q=70&w=400) Cardiac MR of a 59-year old patient carrying an implantable cardioverter defibrillator (ICD). The cine steady state with free precession sequence (time of repetition [TR] 50 ms, time to echo [TE] 3.3 ms, flip angle 80°) acquired along the four-chamber view (a) was largely impaired by a large concentric artifact (asterisk in a) not allowing for segmenting both ventricles. The corresponding cine gradient echo fast low-angle shot sequence (b) (TR 49 ms, TE 1.5 ms, flip angle 15°) shows a strongly reduced artifact generated by the ICD not impairing the evaluation of both ventricles. The artifact generated by the electro-catheter is visible in the right atrium in both images (white arrows in a and b). Image courtesy of Moreno Zanardo.
Cardiac MR of a 59-year old patient carrying an implantable cardioverter defibrillator (ICD). The cine steady state with free precession sequence (time of repetition [TR] 50 ms, time to echo [TE] 3.3 ms, flip angle 80°) acquired along the four-chamber view (a) was largely impaired by a large concentric artifact (asterisk in a) not allowing for segmenting both ventricles. The corresponding cine gradient echo fast low-angle shot sequence (b) (TR 49 ms, TE 1.5 ms, flip angle 15°) shows a strongly reduced artifact generated by the ICD not impairing the evaluation of both ventricles. The artifact generated by the electro-catheter is visible in the right atrium in both images (white arrows in a and b). Image courtesy of Moreno Zanardo."Considering 6,237 MRI exams in 5,394 device-dependent or nondependent carriers of conditional or nonconditional ICDs, a very low rate of clinically relevant adverse events was reported. The risk/benefit ratio is largely positive, also for nonconditional ICDs," the authors wrote.
The scientific posters from the ESCR 2017 meeting are available in the European Society of Radiology's EPOS database. To view the exhibit by Zanardo and colleagues, click here. To view the poster by Lastella and colleagues, click here.